The global population is facing an unprecedented ocular health crisis driven by the proliferation of digital screens. Daily engagement with smartphones, tablets, and computer monitors has shifted human visual focus overwhelmingly to near-field distances, creating a relentless strain on the eye’s accommodation system. Epidemiological data confirms that average daily smartphone usage now surpasses three hours, frequently climbing to six hours or more when combined with professional computer use. This sustained close-up visual environment is strongly correlated with a constellation of negative health outcomes collectively known as digital eye strain, including chronic dry eye syndrome, ocular fatigue, intermittent blurred vision, and refractory headaches. Furthermore, this intense near-focus burden is implicated in the accelerated progression of myopia, particularly among younger demographics.
Against this backdrop of worsening digital dependence, Edenlux, a sophisticated South Korean startup, has positioned itself at the nexus of ophthalmic science, wearable technology, and artificial intelligence. Headquartered in Seoul, the company is not merely developing another consumer gadget; it is engineering a comprehensive technological response to the systemic eye and auditory health issues resulting from modern digital lifestyles.
The genesis of Edenlux is rooted in the personal and profound medical crisis of its founder and CEO, Sungyong Park. A trained medical doctor who previously served as a military physician, Park experienced a rare and alarming side effect after receiving a muscle relaxant injection for severe cervical stiffness. This injection induced a temporary, yet debilitating, paralysis of the crucial ocular muscles responsible for the critical function of focusing—a condition that left him without control over his visual accommodation. Facing a medical prognosis that offered little beyond passive waiting for recovery, Park rejected inertia. He proactively sourced specialized ophthalmic diagnostic and training equipment, commencing an intensive, self-directed regimen to retrain and rehabilitate his paralyzed eye muscles. His eventual recovery provided him with an unparalleled, firsthand understanding of the plasticity and rehabilitative potential of the visual system. This transformative personal experience directly catalyzed his pivot from clinical practice to entrepreneurial endeavor, forging the foundational mission of Edenlux: to equip individuals with the tools necessary to actively protect and restore their vision in a world saturated with digital light.
Edenlux is now preparing its strategic entry into the critical U.S. market with its second-generation daily visual recovery tool, Eyeary. The launch, planned for the end of March via the crowdfunding platform Indiegogo, marks a significant shift in design and technological capacity from its predecessor. Crucially, Edenlux’s products are deliberately categorized under the U.S. Food and Drug Administration’s (FDA) general wellness classification. This regulatory pathway permits the devices to be marketed for vision training, general eye health maintenance, and recovery, distinguishing them from regulated medical devices that require rigorous clinical trials for specific therapeutic claims. The decision to utilize Indiegogo, rather than pursue immediate venture capital funding for the launch, was reportedly driven by the company’s strong financial footing, citing sufficient cash reserves—approximately $99 million raised across Series A ($39 million in 2020) and Series B ($60 million in 2022) funding rounds—to sustain operations and product deployment for several years.
The technological precursor to Eyeary was Otus, which debuted in 2022 across several key Asian markets, including South Korea, Singapore, Japan, and Taiwan. Otus, characterized by a bulkier, VR-headset-like form factor, utilized a system of dynamic lenses to precisely control the contraction and relaxation cycles of the ciliary muscle. This initial product successfully generated $10 million in cumulative revenue, validating the market demand for active vision wellness technology. However, the operational complexity and size of Otus presented barriers to widespread daily adoption.
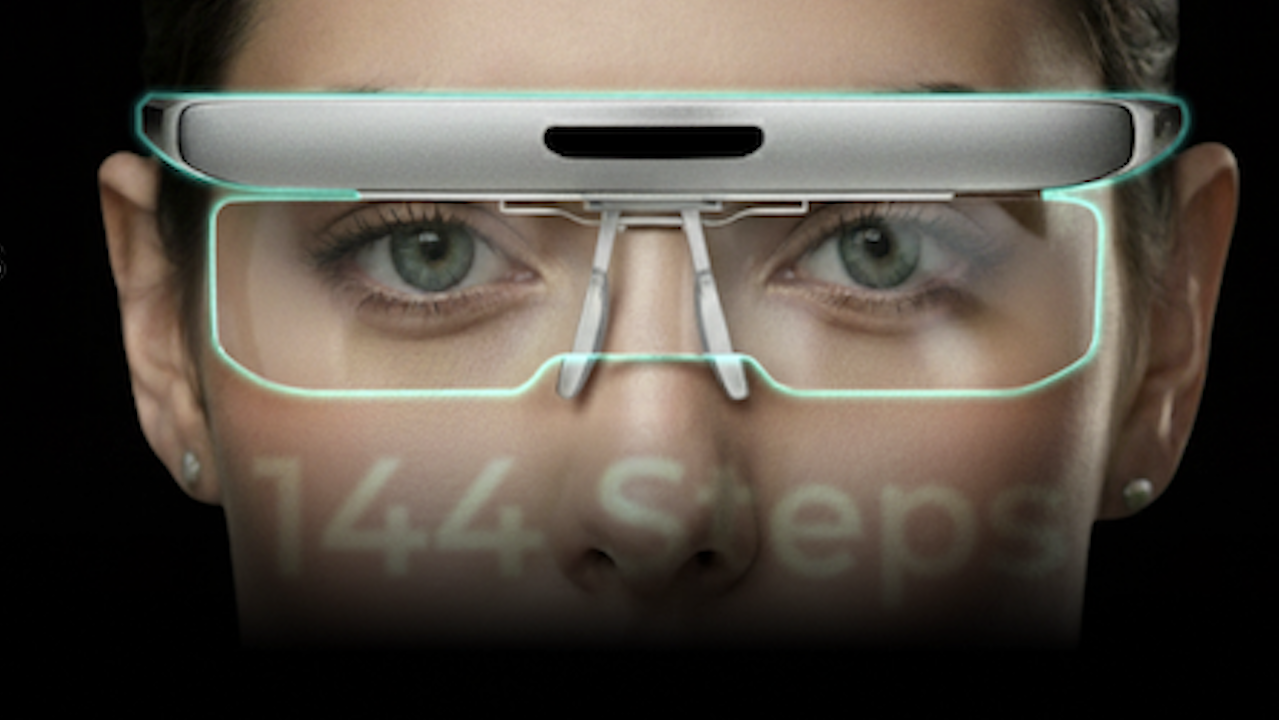
Eyeary represents a monumental leap in form factor and functional sophistication. While Otus offered five discrete diopter focal points for training, Eyeary incorporates a revolutionary lens system featuring 144 diopter focal points. This exponential increase in focusing precision allows for significantly finer and more granular adjustments, enabling highly individualized and precise training of the ciliary muscle complex. This enhanced fidelity is projected to dramatically accelerate recovery timelines. According to CEO Park, where Otus users typically required approximately 12 months of consistent use to reduce reliance on reading glasses, the superior capabilities of Eyeary could potentially halve that duration, achieving similar results in approximately six months.
Beyond the technical advancements, Eyeary has undergone a profound aesthetic transformation. It transitions from the clunky, immersive appearance of a VR device to the sleek, lightweight profile of conventional eyeglasses. This design evolution is crucial for integrating the device seamlessly into the user’s daily routine, encouraging adherence to the prescribed training protocols. The device functions as a sophisticated data collection instrument, pairing via Bluetooth with a dedicated mobile application. This app logs detailed usage metrics, which are then transmitted to Edenlux’s secure cloud servers. The company utilizes these comprehensive datasets—analyzing vision profiles, age demographics, and gender variables—to feed proprietary artificial intelligence algorithms. This AI layer is indispensable, allowing Edenlux to predict personalized improvement timelines and dynamically customize visual training programs, moving the product beyond static exercise regimes toward genuine personalized ophthalmic wellness.

The physiological target of this technology is the ciliary muscle, a critical component of the eye’s accommodation mechanism. This muscle is responsible for changing the shape of the crystalline lens, enabling the eye to shift focus between near and distant objects. Dr. Park emphasizes the mechanism of digital strain: "When people are young, the ciliary muscle is robust enough to handle rapid and frequent focus changes. But constant smartphone use forces the eye into a state of sustained contraction for near-focus. Over prolonged periods, this constant workload leads to muscle fatigue, reduced flexibility, and eventually, chronic vision problems." By providing controlled, repetitive cycles of contraction and relaxation across a broad spectrum of focal distances, Eyeary aims to actively exercise and restore the functional elasticity of the ciliary muscle, mitigating the effects of digital-induced accommodative spasm and presbyopia (age-related reading difficulty).
Edenlux’s strategic roadmap extends far beyond Eyeary. The company is developing a comprehensive suite of digital health solutions, each targeting specific ophthalmic and auditory conditions. This product ecosystem includes Tearmore, designed to address dry eye syndrome; Lux-S, aimed at strabismus (eye misalignment); Lumia, focused on preventative measures against the onset or progression of myopia; and Heary, a device dedicated to auditory recovery, addressing the hearing issues often linked to prolonged earphone use. While Eyeary is leading the U.S. expansion, these specialized devices are slated for initial rollout across Asian markets, demonstrating a global strategy for specialized consumer health technology.
In terms of market positioning, CEO Park views established consumer biometrics companies, such as Oura Ring, as strategic peers. The fundamental commonality lies in the business model: both leverage hardware to collect deep human physiological data and translate that data into actionable insights through software, often via a subscription-based model. However, where companies like Oura focus on foundational systemic metrics like heart rate variability, sleep quality, and body temperature, Edenlux carves out a specialized niche, concentrating on highly specific and underserved biometrics: vision and hearing health. This focus targets the root causes of discomfort and functional degradation resulting from the overuse of personal digital devices—a demographic encompassing virtually all smartphone and earphone users globally.
The industry implications of Edenlux’s successful scaling are substantial. Their approach represents a powerful convergence of preventative medicine and consumer electronics. The transition from the bulky Otus to the cosmetically appealing Eyeary underscores a significant trend in the wellness technology sector: specialized health devices must achieve a high level of aesthetic integration to gain mass market acceptance. Moreover, the sheer volume of data collected via the 144-diopter system and the associated AI analysis positions Edenlux as a potential leader in predictive ophthalmic health. By aggregating and analyzing data across millions of usage hours, the company could move beyond simple training and start developing predictive models for presbyopia onset, myopia progression rates, and personalized intervention efficacy.
The company’s decision to establish a U.S. subsidiary in Dallas, Texas, where final device assembly will take place, is a crucial move for navigating the complex logistics and consumer trust requirements of the North American market. It signifies a commitment to quality control and reduced supply chain vulnerability, while also ensuring closer proximity to a primary target customer base.
Looking forward, Edenlux is actively exploring potential integration partnerships with global technology behemoths such as Apple and Samsung. The ultimate vision is to embed its proprietary vision-protecting technology directly into the core functionality of future smartphones and augmented reality headsets. If successful, this integration would shift the paradigm from reactive wellness devices to proactive, built-in digital health features, fundamentally changing how billions of users interact with their screens and mitigating digital strain at the source.
The launch of Eyeary in the U.S. is not merely a product debut; it is a declaration that eye health in the digital age is rapidly evolving from a niche concern into a central pillar of consumer technology. By merging medical expertise, substantial venture capital backing, sophisticated data science, and consumer-friendly hardware, Edenlux is pioneering a new category of specialized preventative health technology, challenging the passive acceptance of vision degradation caused by our screen-centric world and offering a data-driven path toward visual recovery and sustained health. This trajectory signals a broader trend where the ‘quantified self’ movement is expanding into highly specialized physiological domains, paving the way for personalized, precise, and preemptive health interventions delivered through daily wearable technology.